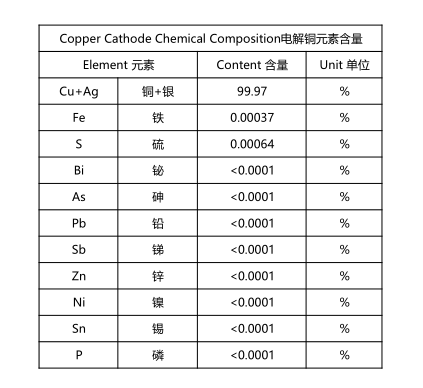
Cathode Copper
A thick plate of crude copper (99% copper) was prepared in advance as the anode, a thin plate of pure copper was prepared as the cathode, and a mixture of sulfuric acid and copper sulfate was used as the electrolyte.After electricity, copper dissolves into copper ions (Cu) from the anode and moves to the cathode, where electrons are obtained and pure copper (also known as electrolytic copper) is precipitated.Impurities in crude copper such as iron and zinc, which are more active than copper, dissolve with copper into ions (Zn and Fe).
Cathode copper usually standard: 99.9 and 99.99 above.